New Crown Antigen Quick Test Nose Kit (Professional Edition)
Novel coronavirus antigen rapid test kit
For professional use only.
For in vitro diagnostics only.
[Intended use]
The novel coronavirus antigen rapid test kit is a lateral flow immunoassay used to qualitatively detect SARS-CoV-2 nucleocapsid antigens in nasopharyngeal swabs and oropharyngeal swabs from people suspected of being COVID-19 by medical institutions. The results can be used to SARS-CoV-2 nucleocapsid antigen identification. In the acute phase of infection, antigens are usually detected in nasopharyngeal swabs and oropharyngeal swabs. A positive result indicates the presence of viral antigens, but the clinical correlation with medical history and other diagnostic information is a necessary condition for determining the status of infection. Positive results do not exclude bacterial infection or co-infection with other viruses. The antigen detected may not be the exact cause of the disease. A negative result does not exclude SARS-CoV-2 infection and should not be the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should take into account the patient's recent exposure, medical history, and clinical symptoms consistent with COVID-19, and can be confirmed by molecular analysis if necessary.
The COVID-19 Antigen Rapid Test Cartridge is intended for use by trained clinical laboratory personnel with specially directed and trained in vitro diagnostic procedures.
[Abstract]
The novel coronavirus (SARS-CoV-2) belongs to the genus β. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. At present, patients with novel coronavirus infection are the main source of infection, and asymptomatic patients may also be the source of infection. According to the current epidemiological survey, the incubation period is 1~14 days, mostly 3~7 days. The main manifestations were fever, fatigue and dry cough. A few cases have nasal congestion, runny nose, sore throat, myalgia and diarrhoea.
[Principle]
COVID-19 antigen rapid detection kit is an immunoassay method based on the principle of double antibody sandwich technology. The COVID-19 antigen rapid test cassette is designed for the detection of SARS-CoV-2 nucleocapsid antigens from nasopharyngeal and oropharyngeal swabs of patients suspected of being COVID-19 by a medical facility. During the test, the sample is moved upwards by capillary action. The SARS-CoV-2 antigen, if present in the specimen, will bind to the antibody conjugate. The immune complex is then captured on the membrane by the pre-coated SARS-Co-2 nucleic acid coating protein monoclonal antibody, and a visible colored line appears in the test line area, indicating a positive result. In the absence of SARS-CoV-2 antigen, the test line area does not form a colored line, indicating a negative result.
As a program control, a colored line will always appear in the control line area, indicating that the appropriate volume of sample has been added and a membrane core has appeared.
[Warnings and Precautions]
• For in vitro diagnostics only.
· Targeting healthcare professionals and nursing professionals.
· Do not use this product as the sole basis for diagnosing or excluding SARS-CoV-2 infection or informing the COVID-19 of infection.
· Do not use after expiration.
· Please read all the information in this manual before performing the test.
· Before use, the test box should be stored in a sealed bag.
· All specimens should be considered as potentially dangerous and treated in the same way as the source of infection.
· Used test boxes should be discarded in accordance with local regulations.
[Composition]
The test cassette comprises a membrane strip coated with an anti-SARS-CoV-2 nucleic acid coat protein monoclonal antibody on a T assay line and a dye pad containing colloidal gold conjugated to a SARS-CoV-2 nucleic acid coat protein monoclonal antibody.
Materials provided
& Yuml; Test box & Yuml; Disinfection cotton swab dropper extraction reagent & Yuml; Instruction
Materials required but not provided
& Yuml; Timer
[Storage and Stability]
• Store in sealed bags at temperature (4-30°C or 40-86 °F). The kit is stable within the validity period on the label. After opening the bag, the test should be used within one hour. Prolonged exposure to hot and humid environments can cause product deterioration.
The lot number and expiration date are printed on the label.
[Sample]
Samples obtained early in the onset of symptoms will contain the highest viral titers; samples obtained five days after the onset of symptoms are more likely to produce a negative result than RT-PCR testing. Inadequate sample collection, improper sample handling and/or transportation may produce false negative results; therefore, training on sample collection is strongly recommended, as sample quality is very important for generating accurate test results
Nasopharyngeal swab sample
A small pointed cotton swab with a flexible rod (wire or plastic) is inserted through the nostril parallel to the palate (not upward) until resistance is encountered or the distance is equal to the distance from the ear to the patient's nostril, indicating contact with the nasopharynx. The depth of the swab should be equal to the distance from the nostril to the external opening of the ear. Gently wipe the swab. The swab is left for a few seconds to absorb the secretions. As you spin, slowly remove the swab. Specimens can be collected from both sides using the same cotton swab, but there is no need to collect specimens from both sides if the microtip is filled with the liquid collected for the first time. If the septum is deflected or blocked making it difficult to obtain a specimen from one nostril, use the same swab to obtain a specimen from the other nostril.
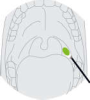
Pharyngeal Swab Samples Swabs were inserted into the posterior pharyngeal and tonsillar areas. Wipe in the tonsil column and posterior oropharynx, avoiding contact with the tongue, teeth and gums.
Sample Preparation
After the swab sample is collected, the swab can be stored in the extraction reagent that comes with the kit. It can also be preserved by immersing the swab head in a test tube containing 2 to 3 mL of virus preservation solution (or isotonic salt solution, tissue culture fluid, or phosphate buffer).
Sample Transportation and Storage
Newly collected specimens should be processed as soon as possible, but no later than one hour after specimen collection. Collected specimens should be stored at 2-8°C for no more than 24 hours. Store at -70°C for a long time, but avoid repeated freeze-thaw cycles.
[Sample Preparation]
1. Insert the swab into an extraction tube containing 0.5 mL of extraction reagent. Rotate the swab to the inside of the test tube using a circular motion to roll the sides of the extraction tube to allow the solution to seep out of the swab and reabsorb. Place the swab in the extraction tube for one minute.
2. Pinch the pipette with your fingers and remove the solution from the cotton swab as much as possible. The extracted solution will be used as a test sample.
3. Keep the extraction tube airtight.
Before testing, allow the test device and sample to equilibrate to temperature (15-30°C or 59-86°C) 1. Remove the test cassette from the sealed bag.
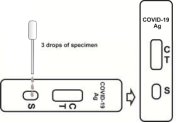
2. Invert the sample extraction tube, stand the sample extraction tube upright, transfer 3 drops (about 100 μL) to the sample hole of the test box, and then start the timer, please refer to the figure below.
3. Wait for the colored lines to appear. The test results were read at 15 minutes. Results are not read after 20 minutes.

[Results explained]
Positive: Two lines appear. One colored line should be in the control area (C), and the other obvious colored line should be in the test area (T). Explain positive for the presence of SARS-CoV-2 nucleocapsid antigens, a positive result indicates the presence of viral antigens, but clinical correlation with the patient's medical history and other diagnostic information is necessary to determine the infection status. A positive result does not exclude bacterial infection or co-infection with other viruses. The detected pathogen may not be the definitive cause of the disease.
Negative: A colored line appears in the control area (C). No line appears in the test area (T). A negative result is presumptive. A negative test result does not exclude infection, nor does it serve as the sole basis for treatment or other patient management decisions, including infection control decisions, especially in patients with clinical signs and symptoms consistent with COVID-19, or exposure to the virus. For patient management, confirmation of these results by molecular detection methods is recommended.
Invalid: The control line cannot be displayed. Insufficient sample size or incorrect processing methods are the most likely causes of control line failure. Review the procedure and repeat the test with a new test cartridge. If the problem persists, stop using the batch immediately and contact your local dealer.
[Quality Control]
test. The colored lines appearing in the control area (C) are considered internal program control. It confirms adequate sample size, adequate membrane core absorption and correct procedure technique.
The kit does not provide a control standard. However, it is recommended that positive and negative controls be tested as good laboratory practices to confirm the test procedure and verify proper test performance.
[Limitations]
• COVID-19 antigen rapid test cartridges are limited to providing qualitative detection. The intensity of the test line does not necessarily correlate with the concentration of the sample antigen.
• A negative result does not exclude SARS-CoV-2 infection and should not be the sole basis for patient management decisions.
• The doctor must interpret the results in conjunction with the patient's medical history, physical examination, and other diagnostic procedures.
• A negative result may occur if the amount of antigen of the SARS-CoV-2 virus present in the sample is below the detection threshold, or if the virus has a slight amino acid mutation in the region of the target epitope recognized by the monoclonal antibody used in the test.
• Proper specimen collection is essential, and failure to follow procedures may produce inaccurate results. Inaccurate results can result from improper sample collection, storage, or repeated freezing and thawing of samples.
[PERFORMANCE CHARACTERISTICS]
Limit of detection (analytical sensitivity)
The detection limit of this kit is 50ng/mL (recombinant SARS-CoV-2 nucleocapsid protein).
Cross-reactivity (analytical specificity)
The cross-reactivity of viral or bacterial cultures at a certain concentration was studied, and when the rapid test was carried out using the COVID-19 antigens, the results were negative:
|
virus/bacteria |
concentration results |
Results |
|
Influenza A (H1N1) |
1 x 106PFU/mL |
- |
|
Influenza A (H3N2) |
1 x 106PFU/mL |
- |
|
Influenza B (Yamagata) |
1 x 106PFU/mL |
- |
|
Flu B (Victoria) |
1 x 106PFU/mL |
- |
|
adenovirus |
1 x 106PFU/mL |
- |
|
human metabolic virus |
1 x 106PFU/mL |
- |
|
parainfluenza virus |
1 x 106PFU/mL |
- |
|
respiratory synchronous virus |
1 x 106PFU/mL |
- |
|
|
1 x 107CFU/mL |
- |
|
|
1 x 107CFU/mL |
- |
|
pneumonia |
1 x 107CFU/mL |
- |





